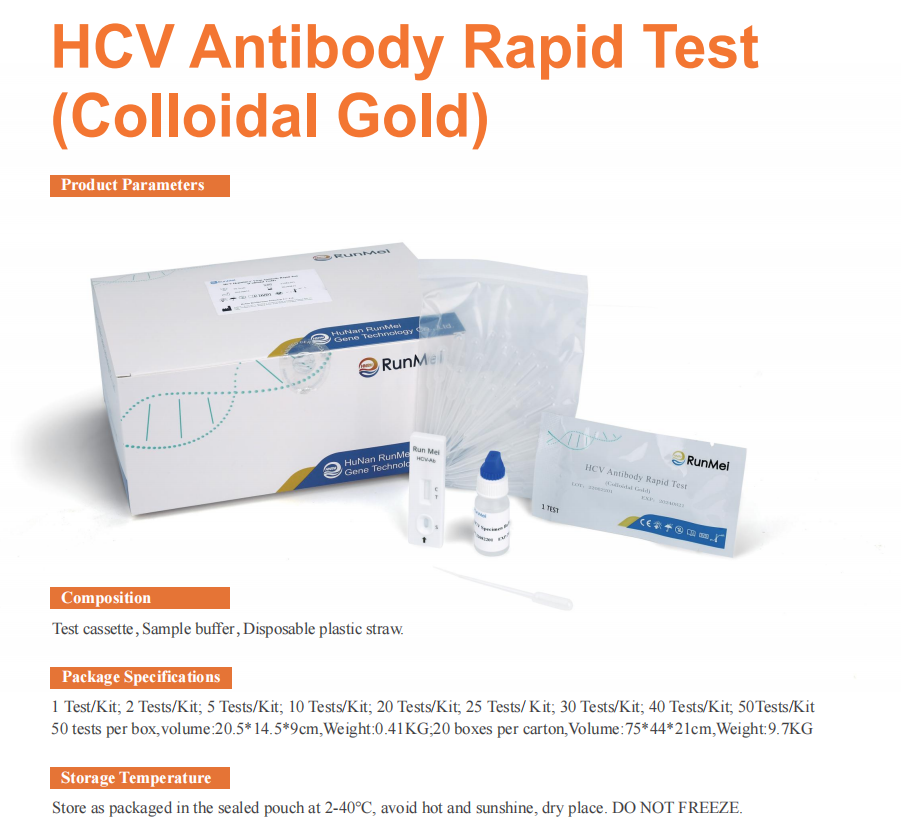
1 Test/Kit; 2 Tests/Kit; 5 Tests/Kit; 10 Tests/Kit; 20 Tests/Kit, 25 Tests/ K it; 30 Tests/Kit; 40 Tests/Kit, 50Tests/Kit
Hunan Runmei Gene
L/C, T/T, D/P, Western Union, Paypal
CE, ISO 13485
Rapid Test Kit / One-step Test Kit / Rapid Detection Test/HCV/Hepatitis C virus
| Availability: | |
|---|---|
CE Marked HCV ( Hepatitis C ) Antibody Rapid Test kit ( Colloidal Gold )
HCV Rapid Antibody Test is a single-use, in vitro diagnostic medical device. It is an immunoassay for the qualitative detection of immunoglobin G (IgG) antibodies to hepatitis C virus (anti-HCV) in oral fluid, fingerstick whole blood, venipuncture whole blood, plasma specimens (EDTA, sodium heparin, lithium heparin, and sodium citrate), and serum (serum separator tube (SST), and from individuals 11 years or older. HCV Rapid Antibody Test assay results may be used to provide presumptive evidence of infection with HCV in individuals with signs and symptoms of hepatitis and in individuals at risk for hepatitis C infection.
Technology: Linear immuno-chromatographic assay
Format: Cassette
Sample type: Whole blood, serum, plasma, oral fluid
Sensitivity: 100%
Specificity: 99.7%
Reading time: 15 minutes
CE Marked HCV ( Hepatitis C ) Antibody Rapid Test kit ( Colloidal Gold )
HCV Rapid Antibody Test is a single-use, in vitro diagnostic medical device. It is an immunoassay for the qualitative detection of immunoglobin G (IgG) antibodies to hepatitis C virus (anti-HCV) in oral fluid, fingerstick whole blood, venipuncture whole blood, plasma specimens (EDTA, sodium heparin, lithium heparin, and sodium citrate), and serum (serum separator tube (SST), and from individuals 11 years or older. HCV Rapid Antibody Test assay results may be used to provide presumptive evidence of infection with HCV in individuals with signs and symptoms of hepatitis and in individuals at risk for hepatitis C infection.
Technology: Linear immuno-chromatographic assay
Format: Cassette
Sample type: Whole blood, serum, plasma, oral fluid
Sensitivity: 100%
Specificity: 99.7%
Reading time: 15 minutes



Assay Procedure of New Coronavirus COVID-19 Antigen rapid test kit
Please read the instruction for use carefully before using this kit. All reagents should be incubated at room temperature (10-30°C) for 30 minutes prior to use. The test should be carried out at room temperature and the operation procedure is described below:
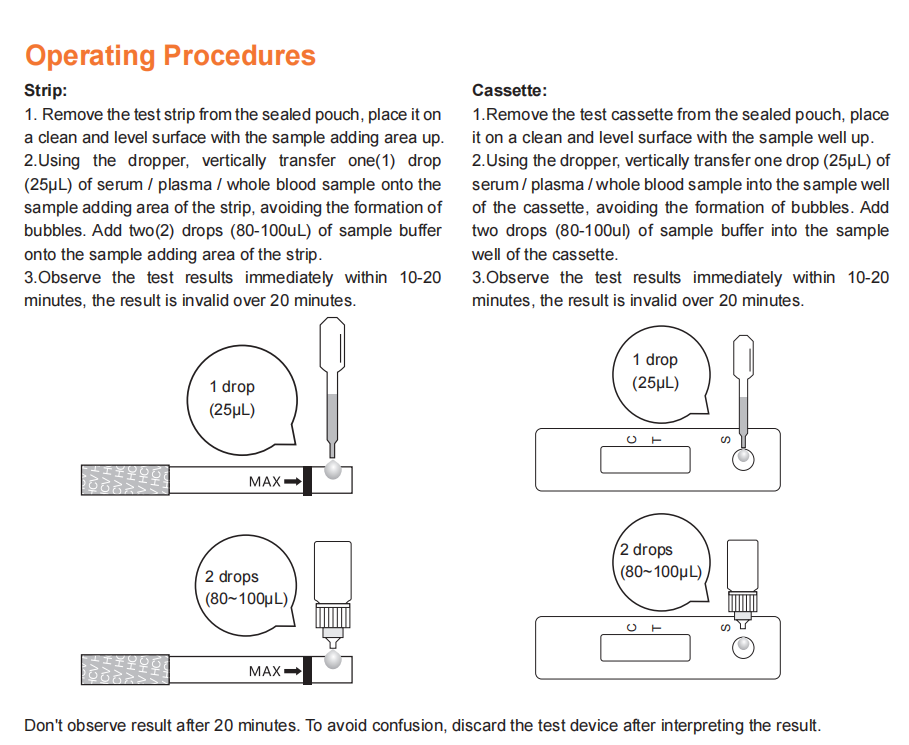
1.Open the sealed bag and remove the Detection Strip. Mark the sample ID on the test strip and lay the strip flat on the table.
2. Specimen collection
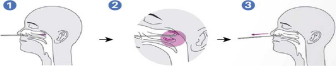
1).Carefully insert the swab into the nostril of the patient, reaching the surface of posterior nasopharynx, that presents the most secretion under visual inspection.
2).Swab over the surface of the posterior nasopharynx. Rotate the swab several times.
3).Withdraw the swab from the nasal cavity.
3.Sample preparation
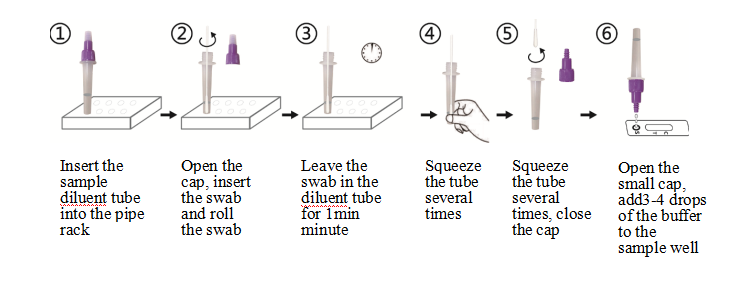
1). Insert the sample diluent tube into the pipe rack, make sure that the tube is standing firm and reaches the bottom of the pipe rack.
2). Open the purple cap of the sample diluent tube. Insert the swab into the diluent tube which contains 0.5 mL of the diluent buffer. Roll the swab at least 6 times while pressing the head against the bottom and side of the diluent tube.
3). Leave the swab in the diluent tube for 1 minute.
4). Squeeze the tube several times with fingers from outside of the tube to immerse the swab. Remove the swab,close the cap. The diluent solution will be used as test sample.
5). Open the small cap on the top of the sample dilution tube. Add 3-4 drops (~100 μL) of the sample diluent buffer immediately to the sample well.
6). Allow the strip to develop for 10-15 minutes at room temperature. A visible band can be read by naked eyes.
Assay Procedure of New Coronavirus COVID-19 Antigen rapid test kit
Please read the instruction for use carefully before using this kit. All reagents should be incubated at room temperature (10-30°C) for 30 minutes prior to use. The test should be carried out at room temperature and the operation procedure is described below:
1.Open the sealed bag and remove the Detection Strip. Mark the sample ID on the test strip and lay the strip flat on the table.
2. Specimen collection
1).Carefully insert the swab into the nostril of the patient, reaching the surface of posterior nasopharynx, that presents the most secretion under visual inspection.
2).Swab over the surface of the posterior nasopharynx. Rotate the swab several times.
3).Withdraw the swab from the nasal cavity.

3.Sample preparation
1). Insert the sample diluent tube into the pipe rack, make sure that the tube is standing firm and reaches the bottom of the pipe rack.
2). Open the purple cap of the sample diluent tube. Insert the swab into the diluent tube which contains 0.5 mL of the diluent buffer. Roll the swab at least 6 times while pressing the head against the bottom and side of the diluent tube.
3). Leave the swab in the diluent tube for 1 minute.
4). Squeeze the tube several times with fingers from outside of the tube to immerse the swab. Remove the swab,close the cap. The diluent solution will be used as test sample.
5). Open the small cap on the top of the sample dilution tube. Add 3-4 drops (~100 μL) of the sample diluent buffer immediately to the sample well.
6). Allow the strip to develop for 10-15 minutes at room temperature. A visible band can be read by naked eyes.
