1 Test/Kit; 2 Tests/Kit; 5 Tests/Kit; 10 Tests/Kit; 20 Tests/Kit, 25 Tests/ K it; 30 Tests/Kit; 40 Tests/Kit, 50Tests/Kit
Hunan Runmei Gene
L/C, T/T, D/P, Western Union, Paypal
CE, ISO 13485
Rapid Test Kit / One-step Test Kit / Rapid Detection Test/SARS-CoV-2+FluA+B+RSV +Adeno Combo Test Kit
| Availability: | |
|---|---|
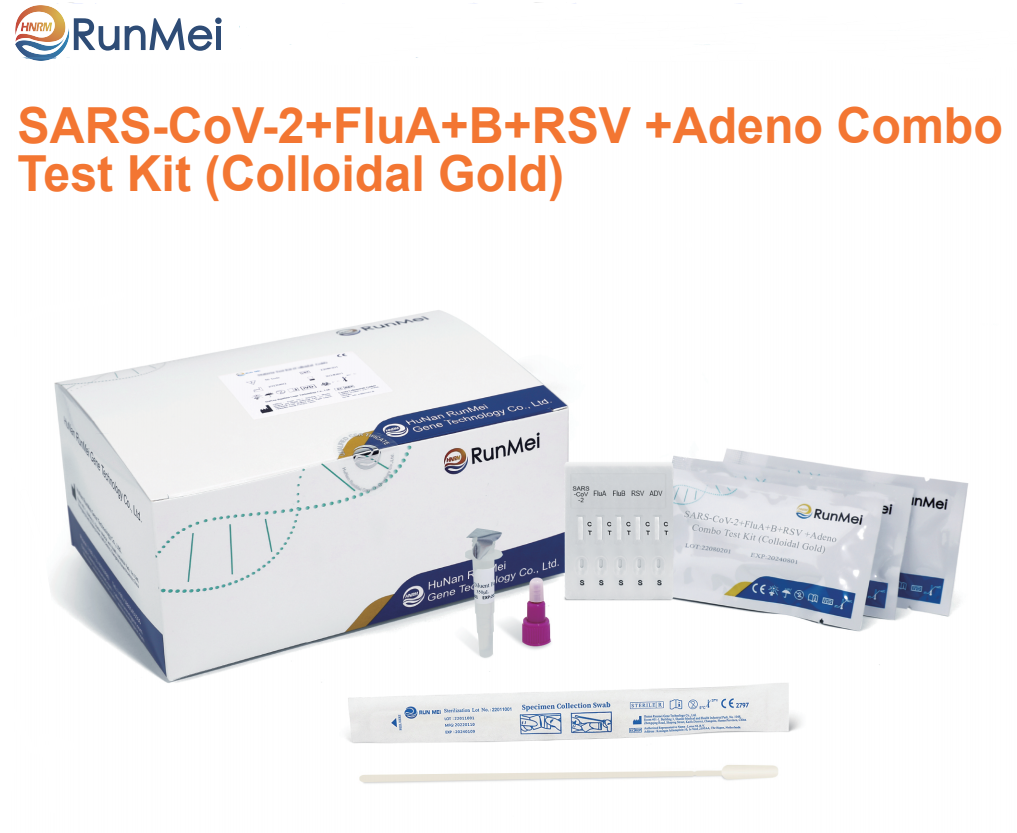
SARS-CoV-2+FluA+B+RSV +Adeno Combo Test Kit (Colloidal Gold)
This kit is a rapid test for the qualitative detection of antigens to SARS-CoV-2,flu A+B, Respiratory syncytial virus and Adenovirus in human nasopharyngeal /oropharyngeal swab specimens or saliva/sputum specimens. This kit is for professional in vitro diagnosis only.
【PRINCIPLE 】
This kit applies the double antibody sandwich assay technology to test the SARS-CoV-2/ influenza A+B / Respiratory syncytial virus/Adenovirus antigens. After dropping a suitable amount of sample onto the sample collection hole, it will move forward alongside the test card. If there is SARS-CoV-2/ influenza A+B/ RSV/ ADV antigens in the specimen, the antigens will bind to colloidal gold labeled SARS-CoV-2/ influenza A+B/ RSV antibodies, and be captured by the another SARS-CoV-2/ influenza A+B/ RSV/ ADV antibodies in the test line, showing a red reaction line, indicating a positive in SARS-CoV-2/ influenza A+B/ RSV/ ADV antigens; otherwise, it is negative. The quality control area (C line) should be red in any circumstances, to indicate that the test is valid, otherwise it is necessary to test the sample again.
Components | 20T | 25T | 40T | 50T |
Test card | 20 pcs | 25 pcs | 40 pcs | 50 pcs |
Disposable sampling tube(with sample diluent buffer) | 20 pcs | 25 pcs | 40 pcs | 50 pcs |
Disposable sampler | 20 pcs | 25 pcs | 40 pcs | 50 pcs |
Instructions | 1 copy | 1 copy | 1 copy | 1 copy |
Note: The components in the kits of different batch numbers are not interchangeable. | ||||
Technology: Linear immuno-chromatographic assay
Format: Cassette
Sample type: Whole blood, serum, plasma, oral fluid
Sensitivity: 100%
Specificity: 99.7%
Reading time: 15 minutes
Results available in only 10 minutes
Easy to use
Results can be read visually
No need for an analyser


SARS-CoV-2+FluA+B+RSV +Adeno Combo Test Kit (Colloidal Gold)
This kit is a rapid test for the qualitative detection of antigens to SARS-CoV-2,flu A+B, Respiratory syncytial virus and Adenovirus in human nasopharyngeal /oropharyngeal swab specimens or saliva/sputum specimens. This kit is for professional in vitro diagnosis only.
【PRINCIPLE 】
This kit applies the double antibody sandwich assay technology to test the SARS-CoV-2/ influenza A+B / Respiratory syncytial virus/Adenovirus antigens. After dropping a suitable amount of sample onto the sample collection hole, it will move forward alongside the test card. If there is SARS-CoV-2/ influenza A+B/ RSV/ ADV antigens in the specimen, the antigens will bind to colloidal gold labeled SARS-CoV-2/ influenza A+B/ RSV antibodies, and be captured by the another SARS-CoV-2/ influenza A+B/ RSV/ ADV antibodies in the test line, showing a red reaction line, indicating a positive in SARS-CoV-2/ influenza A+B/ RSV/ ADV antigens; otherwise, it is negative. The quality control area (C line) should be red in any circumstances, to indicate that the test is valid, otherwise it is necessary to test the sample again.
Components | 20T | 25T | 40T | 50T |
Test card | 20 pcs | 25 pcs | 40 pcs | 50 pcs |
Disposable sampling tube(with sample diluent buffer) | 20 pcs | 25 pcs | 40 pcs | 50 pcs |
Disposable sampler | 20 pcs | 25 pcs | 40 pcs | 50 pcs |
Instructions | 1 copy | 1 copy | 1 copy | 1 copy |
Note: The components in the kits of different batch numbers are not interchangeable. | ||||
Technology: Linear immuno-chromatographic assay
Format: Cassette
Sample type: Whole blood, serum, plasma, oral fluid
Sensitivity: 100%
Specificity: 99.7%
Reading time: 15 minutes
Results available in only 10 minutes
Easy to use
Results can be read visually
No need for an analyser



Assay Procedure of New Coronavirus COVID-19 Antigen rapid test kit
Please read the instruction for use carefully before using this kit. All reagents should be incubated at room temperature (10-30°C) for 30 minutes prior to use. The test should be carried out at room temperature and the operation procedure is described below:
1.Open the sealed bag and remove the Detection Strip. Mark the sample ID on the test strip and lay the strip flat on the table.
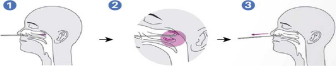
2. Specimen collection
1).Carefully insert the swab into the nostril of the patient, reaching the surface of posterior nasopharynx, that presents the most secretion under visual inspection.
2).Swab over the surface of the posterior nasopharynx. Rotate the swab several times.
3).Withdraw the swab from the nasal cavity.
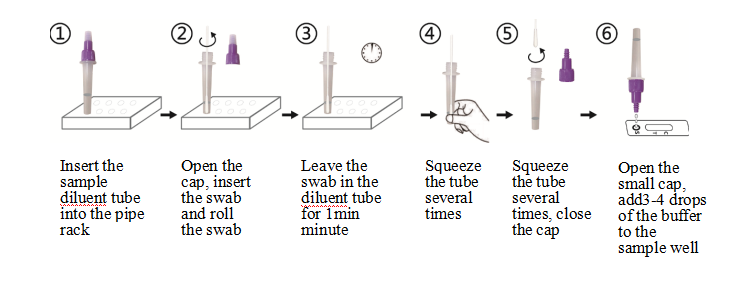
3.Sample preparation
1). Insert the sample diluent tube into the pipe rack, make sure that the tube is standing firm and reaches the bottom of the pipe rack.
2). Open the purple cap of the sample diluent tube. Insert the swab into the diluent tube which contains 0.5 mL of the diluent buffer. Roll the swab at least 6 times while pressing the head against the bottom and side of the diluent tube.
3). Leave the swab in the diluent tube for 1 minute.
4). Squeeze the tube several times with fingers from outside of the tube to immerse the swab. Remove the swab,close the cap. The diluent solution will be used as test sample.
5). Open the small cap on the top of the sample dilution tube. Add 3-4 drops (~100 μL) of the sample diluent buffer immediately to the sample well.
6). Allow the strip to develop for 10-15 minutes at room temperature. A visible band can be read by naked eyes.
Assay Procedure of New Coronavirus COVID-19 Antigen rapid test kit
Please read the instruction for use carefully before using this kit. All reagents should be incubated at room temperature (10-30°C) for 30 minutes prior to use. The test should be carried out at room temperature and the operation procedure is described below:
1.Open the sealed bag and remove the Detection Strip. Mark the sample ID on the test strip and lay the strip flat on the table.
2. Specimen collection
1).Carefully insert the swab into the nostril of the patient, reaching the surface of posterior nasopharynx, that presents the most secretion under visual inspection.
2).Swab over the surface of the posterior nasopharynx. Rotate the swab several times.
3).Withdraw the swab from the nasal cavity.

3.Sample preparation
1). Insert the sample diluent tube into the pipe rack, make sure that the tube is standing firm and reaches the bottom of the pipe rack.
2). Open the purple cap of the sample diluent tube. Insert the swab into the diluent tube which contains 0.5 mL of the diluent buffer. Roll the swab at least 6 times while pressing the head against the bottom and side of the diluent tube.
3). Leave the swab in the diluent tube for 1 minute.
4). Squeeze the tube several times with fingers from outside of the tube to immerse the swab. Remove the swab,close the cap. The diluent solution will be used as test sample.
5). Open the small cap on the top of the sample dilution tube. Add 3-4 drops (~100 μL) of the sample diluent buffer immediately to the sample well.
6). Allow the strip to develop for 10-15 minutes at room temperature. A visible band can be read by naked eyes.
